Specific Heat Capacity Units
Heat capacity or thermal capacity is a physical property of matter defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The specific gas constant is very useful in engineering applications of thermodynamics.

Physics Ch 0 5 Standard Units 12 Of 41 Specific Heat And Molar Heat Capacity Youtube
Specific heat of Sand is 830 Jg K.
. The constant pressure of the specific heat capacity of steam is 18723 kJkg K. The table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and engineering materials and when applicable the molar heat capacity. Heat capacity is an extensive propertyThe corresponding intensive property is the specific heat capacity found by dividing the heat capacity of an object.
Units of Heat - BTU Calorie and Joule - The most common units of heat BTU - British Thermal Unit Calorie and Joule. When you find the heat capacity of one unit of something 1 gram 1 ounce 1 kilogram etc youve found this objects specific heat. The intensive properties c v and c p are defined for pure simple compressible substances as partial derivatives of the internal energy uT v and enthalpy hT p respectively.
Not sure which is the best heat pump for your next project. Generally the most constant parameter is notably the volumetric heat capacity at least for solids which is around the value of 3 megajoule per cubic meter per kelvin. 1 joule 1 kgm 2 s -2 0239005736 calorie The specific heat capacity of water vapour at room temperature is also higher than most other materials.
The specific heat power of water is 42 joules per gram per Celsius degree. Give us a call at 888 292-0874 and we can help you find the perfect heat pump to fit your. The Scottish scientist Joseph Black in the 18th century noticed that equal masses of.
The definition of the calorie is based on the specific heat of water defined. Also explore many other unit converters or learn more about specific heat capacity unit conversions. Butane - Specific Heat vs.
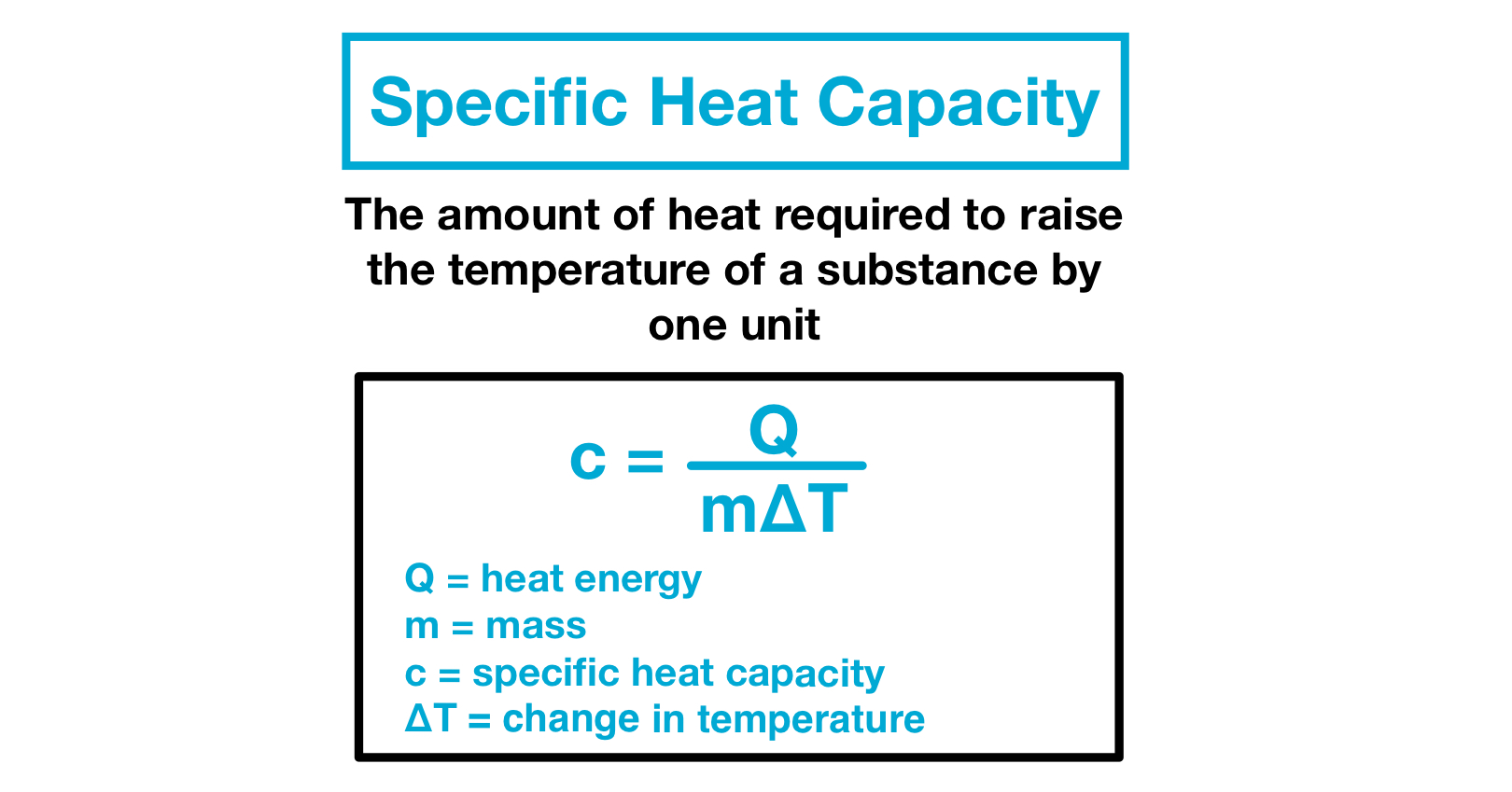
List of thermal conductivities Note that the especially high molar values as for paraffin gasoline water and ammonia result from calculating specific heats in terms of moles of molecules. For example the specific heat of water is 1 calorie or 4186 joules per gram per Celsius degree. The heat needed to raise a substances temperature by 1 degree Celcius is called the specific heat capacity.
Free online specific heat capacity converter - converts between 20 units of specific heat capacity including joulekilogramK JkgK joulekilogramC JkgC joulegramC JgC kilojoulekilogramK etc. Specific heat the quantity of heat required to raise the temperature of one gram of a substance by one Celsius degree. For example it takes 417 Joules to raise.
One calorie 4184 joules. If specific heat is expressed per mole of atoms for these substances none of the constant-volume values exceed to any large extent the theoretical. Temperature - Online calculator figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to 360 C 32-700 F - SI and Imperial units.
The constant volume of the specific heat capacity of steam is 14108 kJkg K. Molar universal Ideal Gas Constant SI unit english units Formula Values Specific Gas Constant 8314 JmolK 0082 LatmmolK 1073 ft3 psilbmolR. The heat capacity in calories per gram is called specific heat.
Water - Specific Heat vs. This 1 calgdeg is the specific heat of the water as a liquid or specific heat capacity of liquid water. The units of specific heat are usually calories or joules per gram per Celsius degree.
It is usually expressed as calories per degree in terms of the actual amount of material being considered most commonly a mole the molecular weight in grams. Table of Specific Heat Capacities. Specific heat or specific heat capacity is a property related to internal energy that is very important in thermodynamics.
Know that specific heat refers to the energy needed to raise one gram by one degree. And c v is the specific heat capacity at constant volume. Use the filters on the left to narrow down your search for your specific heat pump application.
Specific heat tells you the amount of energy needed to raise each unit one degree. The SI unit of heat capacity is joule per kelvin JK. Heat capacity ratio of heat absorbed by a material to the temperature change.
Ammonia - Specific Heat vs. Temperature and Pressure - Online calculator figures and tables showing specific heat C P and C V of gasous and liquid ammonia at temperatures ranging from -73 to 425C -100 to 800F at pressure ranging from 1 to 100 bara 145 - 1450 psia - SI and Imperial Units.
Chem 101 General Chemistry Topic

Specific Heat And Latent Heat Youtube
Heat Capacity Of Water Overview Importance Expii

Temperature Thermodynamics Units In Calculations Of Heat Q Physics Stack Exchange
0 Response to "Specific Heat Capacity Units"
Post a Comment